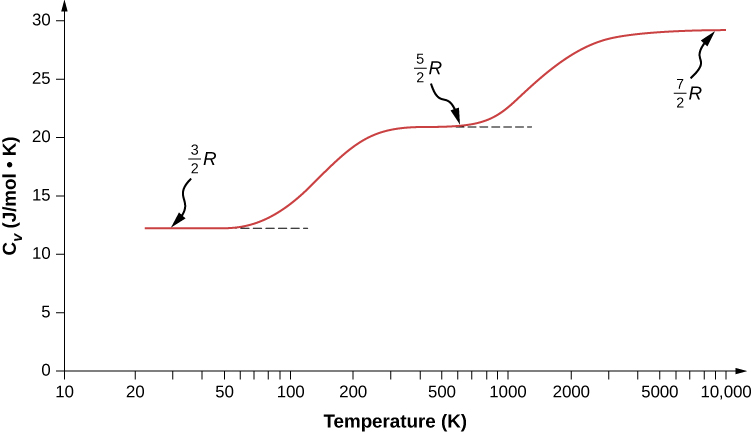
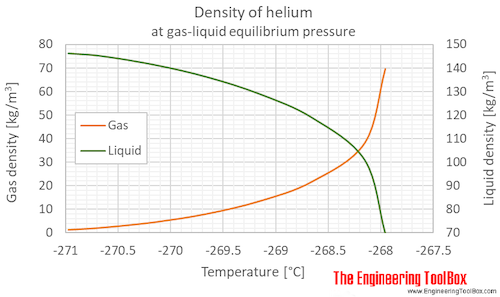
The liquid form of the element is the only liquid known to man that cannot be solidified no matter how low the temperature drops.
Elements that are gases at room temperature and pressure.
Many inorganic and organic compounds with four or fewer nonhydrogen atoms are also gases at room temperature and pressure.
With only rare exception these gases have relatively small molecular weights.
Radon helium xenon neon krypton and argon are eight noble gases.
Elements that exist as gases at room temperature and pressure are clustered on the right side of the periodic table.
Elements in the noble gas group.
The elements that are gases at room temperature include hydrogen.
They also include all the inert or noble gases which are those elements in group 18.
Compounds that are gases at room temperature are all covalent compounds such as co 2 so 2 and nh 3 that contain two or more nonmetals.
There are 7 diatomic elements but only 5 diatomic elements at standard temperature and pressure.
Elements that are liquid at 25 c.
Include both elements and compounds.
Compounds that are gases at room temperature are all covalent compounds.
With a few exceptions gases at room temperature have relatively small molar masses.
Room temperature is a loosely defined term that can mean anywhere from 20 c to 29 c.
Patterns to common gases at room temperature.
Helium neon argon krypton xenon and radon.
Mercury hg and bromine br are the only elements in the periodic table that are liquids at room temperature.
Helium is so light it can escape the atmosphere and bleed away into space.
The following 5 element gases are found as diatomic molecules at room temperature and pressure.
In molecular fluorine f 2 the atoms are held together by a bond made from the union of a p orbital from each atom with such a bond being classed as a sigma bond.
Each of the 13 elements has their own unique physical and chemical properties.
For science it s usually considered to be either 20 c or 25 c.
Hydrogen h 2.
At room temperature and atmospheric pressure the halogen elements in their free states exist as diatomic molecules.
They are nonreactive mono atomic elements with extremely low boiling points.
Photographs and descriptions of many samples from the collection gas at room temperature in the periodic table.
The diatomic elements are hydrogen nitrogen oxygen fluorine chlorine bromine and iodine.
Elements that are gases at room temperature are all nonmetals.
Gas at room temperature 13 these elements are gasses at room temperature and pressure.
At this temperature and ordinary pressure only two elements are liquids.
They occur as either monatomic gases the noble gases or diatomic molecules some halogens n 2 o 2.